How do Lithium Ion Batteries Work: A Comprehensive Overview
Lithium ion batteries have become an integral part of our daily lives, powering everything from our smartphones to electric vehicles. But have you ever wondered how these compact powerhouses actually work? In this article, we will delve into the fascinating world of lithium ion batteries, exploring their inner workings and shedding light on the science behind their exceptional performance.
Lithium ion batteries are a type of rechargeable battery that store and release electrical energy through the movement of lithium ions between two electrodes, an anode and a cathode. This electrochemical process enables the battery to generate a steady flow of electricity, providing a reliable source of power for a wide range of applications.
At the heart of a lithium ion battery is the electrolyte, a conductive material that allows the movement of lithium ions between the electrodes. Typically, the electrolyte is a liquid or gel-like substance that contains lithium salts, which act as the carrier for the ions. When the battery is charged, lithium ions are extracted from the cathode and move through the electrolyte to the anode, where they are stored. During discharge, the ions flow back to the cathode, generating an electric current.
One of the key advantages of lithium ion batteries is their high energy density, which allows them to store a large amount of energy in a compact and lightweight package. This makes them ideal for portable devices where space and weight are critical factors. Additionally, lithium ion batteries have a low self-discharge rate, meaning they can hold their charge for extended periods without significant loss of energy.
In conclusion, lithium ion batteries are a marvel of modern technology, providing us with a reliable and efficient source of power. In the following sections, we will explore the various components of lithium ion batteries in more detail, including the anode, cathode, and electrolyte, as well as the charging and discharging processes. So, let’s dive deeper into the world of lithium ion batteries and uncover the secrets behind their impressive performance.
How Do Lithium Ion Batteries Work?
Lithium-ion batteries have become an integral part of our lives, powering everything from smartphones to electric vehicles. But have you ever wondered how these compact powerhouses actually work? In this article, we will delve into the intricate workings of lithium-ion batteries and explore the science behind their efficiency and reliability.
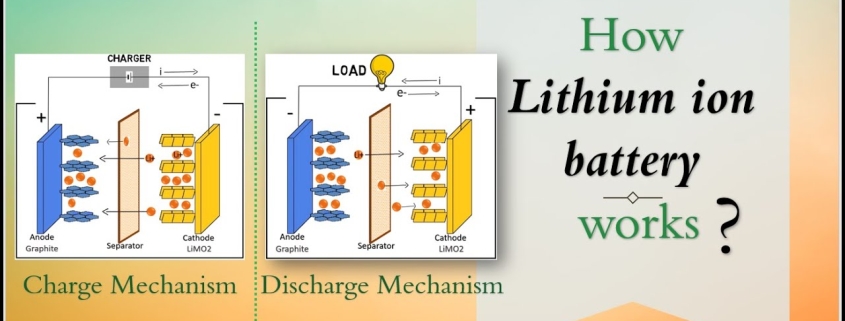
At the heart of a lithium-ion battery are two electrodes: a cathode and an anode. The cathode is typically made of lithium cobalt oxide, while the anode is usually composed of graphite. These electrodes are separated by a thin layer called the electrolyte, which allows the movement of lithium ions between the electrodes.
When the battery is being charged, lithium ions move from the cathode to the anode through the electrolyte. This process is facilitated by an external power source, such as a charger. The movement of ions creates a flow of electrons, which generates an electric current that charges the battery.
During discharge, the process is reversed. The lithium ions move from the anode back to the cathode, releasing energy in the form of electrical power. This energy can then be used to power various devices.
One of the key advantages of lithium-ion batteries is their high energy density. This means they can store a significant amount of energy in a compact size, making them ideal for portable devices. Additionally, lithium-ion batteries have a low self-discharge rate, meaning they can hold their charge for extended periods without losing power.
In conclusion, the workings of lithium-ion batteries are complex yet fascinating. Understanding how these batteries operate allows us to appreciate their importance in our daily lives. Whether it’s powering our smartphones or enabling the adoption of electric vehicles, lithium-ion batteries have revolutionized the way we store and use energy.
What Are the Components of a Lithium Ion Battery?
Lithium-ion batteries have become an integral part of our daily lives, powering everything from smartphones to electric vehicles. But have you ever wondered what makes these batteries work? In this article, we will explore the components that make up a lithium-ion battery and how they work together to store and release energy efficiently.
The key components of a lithium-ion battery include the cathode, anode, electrolyte, and separator. The cathode is typically made of a metal oxide, such as lithium cobalt oxide or lithium iron phosphate. It acts as the positive electrode and is responsible for storing lithium ions during charging. On the other hand, the anode, usually made of graphite, acts as the negative electrode and releases lithium ions during discharging.
The electrolyte is a crucial component that allows the movement of lithium ions between the cathode and anode. It is typically a lithium salt dissolved in an organic solvent. The electrolyte ensures that the lithium ions can flow freely, enabling the battery to charge and discharge efficiently.
To prevent short circuits and maintain the separation between the cathode and anode, a separator is used. This component is usually made of a porous material that allows the passage of lithium ions while preventing direct contact between the electrodes.
When a lithium-ion battery is charged, lithium ions move from the cathode to the anode through the electrolyte, where they are stored. During discharge, the lithium ions move back to the cathode, releasing energy that can be used to power various devices.
Understanding the components of a lithium-ion battery is essential for optimizing their performance and ensuring their longevity. By continuously improving these components, researchers are working towards developing batteries with higher energy densities, longer lifetimes, and faster charging capabilities.
In conclusion, the components of a lithium-ion battery, including the cathode, anode, electrolyte, and separator, work together to store and release energy efficiently. By harnessing the power of these components, we can continue to enjoy the benefits of portable and rechargeable devices in our modern world.
How Does the Charging Process Work?
Charging a lithium-ion battery is a crucial step in ensuring its optimal performance and longevity. Understanding how the charging process works is essential for anyone who uses devices powered by these batteries.
The charging process of lithium-ion batteries involves several key steps. First, the battery is connected to a charger that supplies a specific voltage and current. The charger regulates the charging process to prevent overcharging, which can damage the battery.
During the charging process, lithium ions move from the positive electrode (cathode) to the negative electrode (anode) through an electrolyte. This movement is facilitated by the charger’s voltage, which creates an electric field that drives the ions. The lithium ions are stored in the anode’s porous structure, which allows for efficient storage and release.
As the battery charges, the lithium ions gradually fill the anode, storing energy. The charger continuously monitors the battery’s voltage and current to determine the charging status. Once the battery reaches its maximum charge level, the charger stops supplying current to prevent overcharging.
It is important to note that the charging process is not linear. The battery’s internal resistance and chemical reactions affect the charging speed. Initially, the battery charges rapidly, but as it approaches full capacity, the charging rate slows down. This is known as the “trickle charge” phase, where the charger supplies a lower current to top off the battery.
In conclusion, the charging process of lithium-ion batteries involves the movement of lithium ions from the cathode to the anode, facilitated by an electric field created by the charger. Understanding this process helps users optimize their battery’s performance and ensure its longevity.
What Are the Advantages of Lithium Ion Batteries?
Lithium-ion batteries have become the go-to power source for many electronic devices, and for good reason. These batteries offer numerous advantages over their counterparts, making them a popular choice in various industries. In this article, we will explore the advantages of lithium-ion batteries and why they have become an integral part of our modern lives.
One of the key advantages of lithium-ion batteries is their high energy density. This means that they can store a large amount of energy in a relatively small and lightweight package. As a result, lithium-ion batteries are ideal for portable devices such as smartphones, laptops, and electric vehicles. Their high energy density allows these devices to run for longer periods without the need for frequent recharging.
Another advantage of lithium-ion batteries is their low self-discharge rate. Unlike other rechargeable batteries, lithium-ion batteries lose very little charge when not in use. This means that you can charge them and store them away for extended periods without worrying about them losing their charge. This makes lithium-ion batteries perfect for emergency backup power supplies and devices that are not frequently used.
Furthermore, lithium-ion batteries have a longer lifespan compared to other rechargeable batteries. They can withstand hundreds, if not thousands, of charge and discharge cycles before their performance starts to degrade. This longevity makes lithium-ion batteries a cost-effective choice in the long run, as they do not need to be replaced as frequently as other battery types.
In addition to their high energy density, low self-discharge rate, and long lifespan, lithium-ion batteries also offer fast charging capabilities. They can be charged at a much faster rate compared to other rechargeable batteries, allowing for quick and convenient power top-ups.
In conclusion, lithium-ion batteries provide several advantages that make them the preferred choice for electronic devices. Their high energy density, low self-discharge rate, long lifespan, and fast charging capabilities make them an efficient and reliable power source. As technology continues to advance, it is likely that lithium-ion batteries will play an even bigger role in powering our devices and shaping our future.
What Are the Disadvantages of Lithium Ion Batteries?
Lithium-ion batteries have become the go-to power source for a wide range of devices, from smartphones to electric vehicles. They offer numerous advantages, such as high energy density and long cycle life. However, like any technology, they also have their drawbacks. In this article, we will explore some of the disadvantages of lithium-ion batteries.
One of the main disadvantages of lithium-ion batteries is their high cost. The materials used in these batteries, including lithium, cobalt, and nickel, can be expensive to produce. This cost is often passed on to the consumer, making devices and electric vehicles powered by lithium-ion batteries more expensive than their counterparts.
Another disadvantage is the limited lifespan of lithium-ion batteries. Over time, the capacity of these batteries decreases, leading to reduced performance. This degradation is accelerated by factors such as high temperatures and frequent charging cycles. Eventually, the battery will need to be replaced, adding to the overall cost.
Safety is another concern with lithium-ion batteries. While incidents are rare, there have been cases of lithium-ion batteries catching fire or exploding. This risk is particularly relevant in applications where the batteries are subjected to extreme conditions or physical damage.
Lithium-ion batteries also have limited energy storage compared to other battery technologies. While they offer high energy density, their capacity is still lower than alternatives like solid-state or flow batteries. This limitation restricts their use in applications that require long-lasting power or high energy demands.
In conclusion, while lithium-ion batteries have revolutionized the portable power industry, they are not without their disadvantages. High cost, limited lifespan, safety concerns, and limited energy storage are all factors to consider when choosing a power source. Despite these drawbacks, ongoing research and development aim to address these issues and improve the performance and affordability of lithium-ion batteries.
How Long Do Lithium Ion Batteries Last?
Lithium-ion batteries have become an integral part of our daily lives, powering everything from smartphones to electric vehicles. But have you ever wondered how long these batteries can actually last? In this article, we will delve into the lifespan of lithium-ion batteries and explore the factors that can affect their longevity.
One of the key factors that determine the lifespan of a lithium-ion battery is the number of charge cycles it can endure. A charge cycle is defined as the process of charging a battery from 0% to 100% and then discharging it back to 0%. On average, a lithium-ion battery can handle around 300 to 500 charge cycles before its capacity starts to degrade. However, it’s important to note that this can vary depending on the quality of the battery and how it is used.
Another factor that affects the lifespan of lithium-ion batteries is temperature. High temperatures can accelerate the degradation process, causing the battery to lose its capacity at a faster rate. On the other hand, storing the battery at extremely low temperatures can also have a negative impact on its performance. Therefore, it is crucial to keep lithium-ion batteries within a recommended temperature range to maximize their lifespan.
Furthermore, the rate at which a lithium-ion battery is discharged can also influence its longevity. Discharging the battery at a high rate puts more stress on its cells, causing them to degrade faster. Therefore, it is advisable to use devices or equipment that discharge the battery at a moderate rate to prolong its lifespan.
In conclusion, the lifespan of lithium-ion batteries can vary depending on several factors such as the number of charge cycles, temperature, and discharge rate. By understanding these factors and taking proper care of our batteries, we can ensure that they last as long as possible, maximizing their value and reducing waste.
How Can I Extend the Lifespan of a Lithium Ion Battery?
Lithium-ion batteries have become an integral part of our daily lives, powering our smartphones, laptops, and even electric vehicles. As these batteries play a crucial role in our modern world, it is important to understand how to extend their lifespan and maximize their performance. In this article, we will explore some practical tips to help you get the most out of your lithium-ion battery.
One of the key ways to extend the lifespan of a lithium-ion battery is to avoid extreme temperatures. High temperatures can cause the battery to degrade faster, while extremely low temperatures can reduce its capacity. Therefore, it is important to store and use your devices in a temperature-controlled environment whenever possible.
Another important factor to consider is the charging habits. It is recommended to avoid frequent full discharges and instead, charge your device in short bursts. Lithium-ion batteries perform best when kept between 20% and 80% charge levels. Additionally, it is advisable to avoid leaving your device plugged in for extended periods once it reaches full charge.
Proper storage is also crucial for maintaining the longevity of your battery. If you’re not planning to use your device for an extended period, it is recommended to store it in a cool and dry place with around 50% charge. This helps prevent the battery from discharging completely or being exposed to high temperatures for long periods.
Regular software updates can also contribute to the overall health of your battery. Manufacturers often release updates that optimize battery performance and efficiency. Therefore, it is important to keep your device up to date with the latest software.
By following these simple tips, you can significantly extend the lifespan of your lithium-ion battery. Proper temperature management, charging habits, storage, and software updates are key factors that can help you get the most out of your devices and keep them running smoothly for years to come.
How Should I Dispose of a Lithium Ion Battery?
Lithium-ion batteries have become an integral part of our lives, powering our smartphones, laptops, and electric vehicles. However, when it comes to disposing of these batteries, many people are unsure of the proper method. In this article, we will explore the best practices for disposing of lithium-ion batteries in an environmentally friendly and safe manner.
First and foremost, it is important to note that lithium-ion batteries should never be thrown in the regular trash. These batteries contain toxic chemicals and heavy metals that can be harmful to the environment if not disposed of properly. Instead, they should be recycled.
Many electronics and battery retailers offer recycling programs for lithium-ion batteries. These programs ensure that the batteries are handled and disposed of safely. Additionally, you can check with your local recycling center or municipality to find out if they accept lithium-ion batteries for recycling.
When preparing a lithium-ion battery for recycling, it is important to take some precautions. First, ensure that the battery is fully discharged. This can be done by using the device until the battery is completely drained. Next, place the battery in a plastic bag or cover the terminals with electrical tape to prevent any accidental short-circuiting.
It is worth noting that damaged or swollen lithium-ion batteries should be handled with extra care. These batteries can be more volatile and should be placed in a non-flammable container, such as a metal box, and taken to a recycling center as soon as possible.
In conclusion, proper disposal of lithium-ion batteries is crucial to protect the environment and ensure the safety of those handling them. By following the guidelines mentioned above and recycling these batteries through authorized programs, we can all contribute to a cleaner and greener future.
In conclusion, understanding how lithium ion batteries work is crucial for anyone using electronic devices powered by these batteries. Throughout this post, we have explored various aspects related to the functioning of lithium ion batteries.
We started by delving into the fundamental question of how lithium ion batteries work. We learned that these batteries rely on the movement of lithium ions between two electrodes, creating a flow of electrons that generates electrical energy.
Next, we discussed the components of a lithium ion battery, including the anode, cathode, and electrolyte. Understanding these components is essential for comprehending the inner workings of these batteries.
Moving on, we explored the charging process of lithium ion batteries. We discovered that during charging, lithium ions move from the cathode to the anode, storing energy for later use.
We then examined the advantages and disadvantages of lithium ion batteries. Their high energy density, long lifespan, and lightweight nature make them a popular choice. However, concerns regarding safety, limited lifespan, and environmental impact should also be considered.
To help readers extend the lifespan of their lithium ion batteries, we provided actionable tips. These included avoiding extreme temperatures, not overcharging or fully discharging the battery, and using the correct charger.
Regarding the disposal of lithium ion batteries, we emphasized the importance of proper recycling to prevent environmental harm. Many local recycling centers accept these batteries, ensuring their safe disposal.
Looking ahead, it is worth noting that advancements in lithium ion battery technology are continuously being made. These developments aim to improve energy storage capacity, charging speed, and overall battery performance.
In conclusion, understanding how lithium ion batteries work is essential for maximizing their benefits while minimizing their drawbacks. We hope this post has provided valuable insights and actionable advice for our readers. Thank you for taking the time to read this post, and we encourage you to leave any comments or feedback you may have.

Leave a Reply
Want to join the discussion?Feel free to contribute!