How Do Lithium Batteries Work: A Fascinating Look into the Power Behind Our Devices
Introduction
Lithium batteries have become an integral part of our daily lives. From smartphones to electric vehicles, these compact powerhouses have revolutionized the way we stay connected and move around. But have you ever wondered how these incredible devices actually work?
In this article, we will delve into the inner workings of lithium batteries, exploring the science behind their impressive performance and longevity. By understanding the fundamental principles that govern these energy storage devices, we can gain a deeper appreciation for their impact on modern technology.
Lithium batteries are rechargeable power sources that utilize the movement of lithium ions between two electrodes to generate electrical energy. At the heart of every lithium battery is a lithium-based compound, such as lithium cobalt oxide or lithium iron phosphate, which serves as the cathode. The anode, on the other hand, is typically made of carbon-based materials.
When a lithium battery is charging, lithium ions are extracted from the cathode and move through an electrolyte solution to the anode. This process is facilitated by an external power source, such as a charger. During discharge, the lithium ions flow back to the cathode, releasing electrical energy that can be harnessed to power our devices.
One of the key advantages of lithium batteries is their high energy density, which allows them to store a significant amount of energy in a compact size. This makes them ideal for portable electronic devices, where space is often limited. Additionally, lithium batteries have a low self-discharge rate, meaning they can retain their charge for extended periods, making them highly efficient and reliable.
In the following sections, we will explore various aspects of lithium batteries in more detail, including their different types, charging and discharging processes, and the environmental impact of their widespread use. By the end of this article, you will have a comprehensive understanding of how lithium batteries work and the role they play in powering our modern world.
So, let’s embark on this illuminating journey into the world of lithium batteries, where science meets innovation and power knows no bounds.
How Do Lithium Batteries Work?
Lithium batteries have become an integral part of our lives, powering everything from smartphones to electric vehicles. But have you ever wondered how these small devices can store so much energy? In this article, we will delve into the inner workings of lithium batteries and explore the science behind their impressive performance.
At the heart of a lithium battery is a chemical reaction that allows for the movement of ions between two electrodes. The electrodes are typically made of lithium compounds, such as lithium cobalt oxide and lithium iron phosphate. These compounds have high energy densities, making them ideal for storing large amounts of energy.
When a lithium battery is charged, lithium ions are extracted from the positive electrode and move through an electrolyte towards the negative electrode. This movement of ions creates a flow of electrons, which is the electric current that powers our devices. During discharge, the process is reversed, with lithium ions moving back to the positive electrode.
One key advantage of lithium batteries is their high voltage and energy density. This means they can store more energy in a smaller and lighter package compared to other types of batteries. Additionally, lithium batteries have a low self-discharge rate, meaning they can hold their charge for extended periods without significant loss.
To ensure safety and prevent overcharging or overheating, lithium batteries are equipped with a built-in protection circuit. This circuit monitors the voltage and temperature of the battery and can cut off the current if any abnormalities are detected.
In conclusion, lithium batteries work by utilizing a chemical reaction that allows for the movement of ions between two electrodes. Their high energy density, low self-discharge rate, and built-in protection circuit make them a popular choice for a wide range of applications. Understanding the inner workings of lithium batteries can help us appreciate their importance in our increasingly connected world.
What Are the Components of a Lithium Battery?
Lithium batteries have become increasingly popular in recent years due to their high energy density and long lifespan. But have you ever wondered what makes up these powerful batteries? In this article, we will explore the components of a lithium battery and how they work together to provide energy.
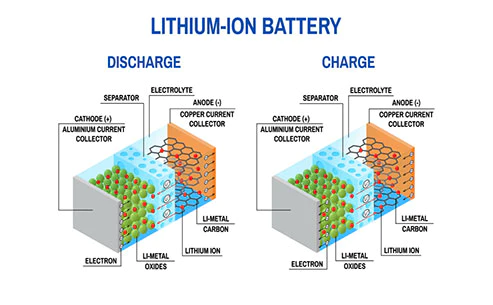
The main components of a lithium battery include the anode, cathode, separator, electrolyte, and collector. The anode is typically made of graphite and is responsible for storing lithium ions during charging. On the other hand, the cathode is made of a metal oxide, such as lithium cobalt oxide, which attracts and stores the lithium ions during discharge.
The separator is a thin, porous material that prevents the anode and cathode from coming into direct contact while allowing the flow of lithium ions. This ensures the battery’s stability and prevents short circuits. The electrolyte, which is usually a liquid or gel, facilitates the movement of lithium ions between the anode and cathode.
Lastly, the collector is responsible for conducting the electrons between the battery and the external circuit. Typically, it is made of a metal such as aluminum or copper and is connected to the anode and cathode.
When a lithium battery is charged, lithium ions move from the cathode to the anode through the electrolyte, while electrons flow through the external circuit, creating a flow of electric current. During discharge, the process is reversed, with lithium ions moving from the anode to the cathode, releasing energy.
In conclusion, the components of a lithium battery work together to store and release energy efficiently. Understanding these components is crucial in harnessing the full potential of lithium batteries for various applications, from portable electronics to electric vehicles.
How Does Lithium-Ion Battery Charging Work?
Lithium-ion batteries have become the go-to power source for a wide range of devices, from smartphones to electric vehicles. But have you ever wondered how these batteries actually work? In this article, we will delve into the intricacies of lithium-ion battery charging and shed light on the science behind it.
The charging process of a lithium-ion battery involves the movement of lithium ions between two electrodes – the cathode and the anode. When the battery is being charged, lithium ions move from the cathode to the anode through an electrolyte solution. This process is facilitated by an external power source, such as a charger.
At the cathode, lithium ions are stored in a layered structure, typically made of lithium cobalt oxide. As the battery charges, the lithium ions are extracted from the cathode and move towards the anode. Meanwhile, the anode is typically made of graphite, which acts as a host for the lithium ions.
During the charging process, the lithium ions are forced into the anode, where they are stored in the graphite structure. This movement of lithium ions is accompanied by a flow of electrons through the external circuit, creating an electric current. Once the battery is fully charged, the movement of lithium ions stops, and the battery is ready to power your device.
It is important to note that lithium-ion batteries require a specific charging voltage and current to ensure safe and efficient charging. Overcharging or charging at a high current can lead to overheating and even fire hazards. Therefore, it is crucial to use a charger that is compatible with your device and follow the manufacturer’s guidelines for charging.
In conclusion, the charging process of lithium-ion batteries involves the movement of lithium ions between the cathode and anode. Understanding this process is essential for safe and efficient charging, ensuring that your devices are powered up and ready to go.
What Are the Advantages of Lithium Batteries?
Lithium batteries have become the go-to power source for a wide range of devices, from smartphones and laptops to electric vehicles and renewable energy storage systems. But what makes them so popular? In this article, we will explore the advantages of lithium batteries and why they are the preferred choice in today’s technology-driven world.
One of the key advantages of lithium batteries is their high energy density. This means that they can store a large amount of energy in a relatively small and lightweight package. Compared to other types of batteries, such as lead-acid or nickel-cadmium batteries, lithium batteries offer a much higher energy density, making them perfect for portable devices that require long-lasting power.
Another advantage of lithium batteries is their long cycle life. Cycle life refers to the number of charge and discharge cycles a battery can go through before its capacity significantly degrades. Lithium batteries have an impressive cycle life, allowing them to be charged and discharged hundreds, if not thousands, of times without losing much capacity. This makes them highly durable and cost-effective in the long run.
In addition, lithium batteries have a low self-discharge rate. Unlike some other battery chemistries, lithium batteries lose very little charge when not in use. This makes them ideal for applications where the battery may sit idle for extended periods, such as emergency backup systems or seasonal devices.
Furthermore, lithium batteries have a fast charging capability. They can be charged at a much faster rate compared to other types of batteries, saving valuable time for users. This is especially important in today’s fast-paced world where people are constantly on the go and need their devices to be ready in no time.
To sum up, the advantages of lithium batteries are their high energy density, long cycle life, low self-discharge rate, and fast charging capability. These features make them the preferred choice for a wide range of applications, providing efficient and reliable power for our modern-day devices.
What Are the Disadvantages of Lithium Batteries?
Lithium batteries have gained significant popularity in recent years due to their high energy density and long lifespan. However, like any other technology, they are not without their drawbacks. Understanding the disadvantages of lithium batteries is essential for making informed decisions about their use.
One of the primary disadvantages of lithium batteries is their high cost. The materials used in their construction, such as lithium cobalt oxide or lithium iron phosphate, can be expensive to produce. This cost is often passed on to the consumer, making lithium batteries more expensive than other types of batteries.
Another disadvantage is their limited lifespan. While lithium batteries have a longer lifespan compared to traditional batteries, they do degrade over time. The capacity of a lithium battery decreases with each charge-discharge cycle, eventually rendering it unusable. This degradation is influenced by factors such as temperature, depth of discharge, and charging rate.
Lithium batteries also have safety concerns. Although rare, they can be prone to thermal runaway, which can lead to overheating, fires, or even explosions. This risk is particularly relevant when lithium batteries are exposed to extreme temperatures or subjected to physical damage.
Furthermore, lithium batteries require careful handling and disposal due to their chemical composition. The lithium-ion cells within these batteries contain toxic materials that can harm the environment if not properly managed. Improper disposal can lead to the release of hazardous substances, posing a risk to human health and the ecosystem.
In conclusion, while lithium batteries offer numerous advantages, it is important to consider their disadvantages as well. High cost, limited lifespan, safety concerns, and environmental impact are some of the drawbacks associated with these batteries. By understanding these disadvantages, individuals and industries can make informed decisions regarding the use and disposal of lithium batteries.
How Long Do Lithium Batteries Last?
Lithium batteries have become an essential part of our daily lives, powering everything from smartphones to electric vehicles. But have you ever wondered how long these batteries can actually last? In this article, we will delve into the lifespan of lithium batteries and explore the factors that can affect their longevity.
One of the key factors that determine the lifespan of a lithium battery is its charge-discharge cycle. Each time a battery is charged and discharged, it goes through a cycle. The number of cycles a battery can endure before its capacity significantly decreases is known as its cycle life. On average, lithium batteries can last anywhere from 300 to 500 cycles, depending on the quality of the battery and how it is used.
Another factor that affects the lifespan of lithium batteries is the rate at which they are charged and discharged. Rapid charging and discharging can cause stress on the battery, leading to a shorter lifespan. It is recommended to charge lithium batteries at a moderate pace to ensure their longevity.
Additionally, the storage conditions of lithium batteries can impact their lifespan. Storing batteries in extreme temperatures, whether too hot or too cold, can cause them to degrade faster. It is best to store lithium batteries in a cool and dry environment to maximize their lifespan.
It’s important to note that the lifespan of lithium batteries can vary depending on the specific application. For example, batteries used in smartphones may have a shorter lifespan compared to those used in electric vehicles. This is because smartphones often go through more charge-discharge cycles on a daily basis.
In conclusion, the lifespan of lithium batteries can range from 300 to 500 cycles, depending on various factors such as charge-discharge cycles, charging rate, and storage conditions. By understanding these factors, we can maximize the lifespan of our lithium batteries and ensure they continue to power our devices efficiently for as long as possible.
How Do Lithium Batteries Compare to Other Types?
Lithium batteries have become increasingly popular in recent years due to their high energy density and long lifespan. But how do they stack up against other types of batteries? In this article, we will compare lithium batteries to some of the most common battery types on the market.
One of the main advantages of lithium batteries is their high energy density. This means that they can store a large amount of energy in a small and lightweight package. Compared to other types of batteries, such as lead-acid or nickel-cadmium batteries, lithium batteries offer a much higher energy density, making them ideal for portable electronic devices like smartphones and laptops.
Another advantage of lithium batteries is their long lifespan. While other types of batteries tend to degrade over time, lithium batteries can last for several years without significant loss in capacity. This is due to the unique chemistry of lithium-ion cells, which allows them to maintain their performance over many charge and discharge cycles.
In addition to their high energy density and long lifespan, lithium batteries also have a fast charging capability. Unlike other types of batteries that may take hours to recharge, lithium batteries can be charged to full capacity in just a fraction of the time. This makes them convenient for users who are always on the go and need their devices to be ready quickly.
However, it’s important to note that lithium batteries also have some limitations. They can be sensitive to high temperatures and can become unstable if not used or stored properly. Additionally, lithium batteries are more expensive to produce compared to other types of batteries, which can make them less cost-effective for certain applications.
In conclusion, lithium batteries offer many advantages over other types of batteries, including high energy density, long lifespan, and fast charging capability. While they may have some limitations, their superior performance makes them the preferred choice for many portable electronic devices. As technology continues to advance, we can expect lithium batteries to become even more efficient and widely used in the future.
What Are the Applications of Lithium Batteries?
Lithium batteries have become increasingly popular due to their high energy density, long lifespan, and lightweight design. These batteries are widely used in various applications, ranging from portable electronics to electric vehicles. Let’s explore some of the key applications of lithium batteries.
One of the most common uses of lithium batteries is in portable electronics such as smartphones, laptops, and tablets. The high energy density of lithium batteries allows these devices to operate for extended periods without requiring frequent recharging. Additionally, lithium batteries can be recharged quickly, making them ideal for on-the-go use.
Another significant application of lithium batteries is in electric vehicles (EVs). The automotive industry is transitioning towards electric mobility, and lithium batteries play a crucial role in powering these vehicles. Lithium batteries offer high energy storage capacity, enabling EVs to achieve longer driving ranges. Furthermore, their lightweight design helps reduce the overall weight of the vehicle, improving its efficiency.
Renewable energy storage is another area where lithium batteries are widely utilized. With the increasing adoption of solar panels and wind turbines, there is a growing need to store excess energy for use during periods of low generation. Lithium batteries provide an efficient and reliable solution for storing renewable energy, allowing for a more stable and sustainable power supply.
Medical devices, such as pacemakers and hearing aids, also benefit from the use of lithium batteries. These batteries offer a long lifespan and consistent power output, ensuring that these critical devices operate reliably for extended periods.
In conclusion, lithium batteries have a wide range of applications, from portable electronics to electric vehicles and renewable energy storage. Their high energy density, long lifespan, and lightweight design make them the preferred choice in many industries. As technology continues to advance, it is likely that the applications of lithium batteries will continue to expand, further revolutionizing various sectors.
In conclusion, this post has provided a comprehensive overview of how lithium batteries work. We have explored the key components of a lithium battery, including the anode, cathode, and electrolyte, and how they interact to store and release energy. Additionally, we have delved into the intricacies of lithium-ion battery charging, understanding the importance of a proper charging process to maximize battery performance and lifespan.
We have also examined the advantages and disadvantages of lithium batteries. Their high energy density, lightweight design, and long lifespan make them a popular choice for various applications. However, their sensitivity to high temperatures and potential for thermal runaway must be carefully managed.
Furthermore, we have discussed the comparison of lithium batteries to other types, such as lead-acid and nickel-cadmium batteries. Lithium batteries outperform these alternatives in terms of energy density, weight, and maintenance requirements.
The applications of lithium batteries are vast and ever-expanding. From powering portable electronic devices to electric vehicles and renewable energy storage systems, lithium batteries have revolutionized numerous industries.
It is important to note that the information provided in this post is timeless and not limited to a specific timeframe. However, it is worth mentioning that the field of lithium battery technology is continuously evolving. Future developments may include advancements in battery capacity, charging speed, and safety measures.
In conclusion, understanding how lithium batteries work is crucial in today’s technology-driven world. By implementing proper charging practices and considering the advantages and disadvantages, users can optimize the performance and lifespan of their lithium batteries.
Thank you for taking the time to read this post. We encourage you to leave any comments or feedback you may have. Stay tuned for future updates on the exciting developments and trends in the world of lithium batteries.



Leave a Reply
Want to join the discussion?Feel free to contribute!