How Do Batteries Work
From powering our smartphones to fueling electric vehicles, batteries play a crucial role in our everyday lives. But have you ever wondered how these small, portable powerhouses actually work? In this article, we will delve into the fascinating world of batteries and uncover the secrets behind their ability to store and release energy.
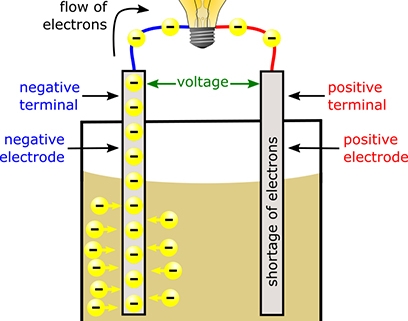
Batteries have been around for centuries, evolving from simple cells to the sophisticated energy storage devices we use today. At their core, batteries consist of three essential components: an anode (negative electrode), a cathode (positive electrode), and an electrolyte. These components work together to create a flow of electrons, generating the electrical energy that powers our devices.
The magic happens through a process called electrochemical reaction. When a battery is connected to a circuit, a chemical reaction occurs within it. At the anode, electrons are released and flow through the circuit, creating an electric current. Meanwhile, at the cathode, a separate chemical reaction takes place, absorbing the electrons and completing the circuit.
The electrolyte, often a liquid or gel, acts as a medium for the movement of ions between the anode and cathode. It allows the flow of charged particles, ensuring a continuous exchange of electrons and maintaining the battery’s functionality.
But how do batteries store energy? This is where the concept of chemical potential energy comes into play. The chemical reactions occurring within the battery create a potential difference, or voltage, between the anode and cathode. This voltage represents the stored energy, which can be released when needed.
In conclusion, batteries are fascinating devices that harness the power of electrochemical reactions to store and release energy. Understanding how they work opens the door to exploring various related topics, such as battery chemistry, different types of batteries, and the future of battery technology. So, let’s embark on this journey together and uncover the secrets of how batteries work. Stay tuned for our upcoming articles that will delve deeper into the world of batteries and their impact on our modern lives.
How Do Batteries Work?
Batteries are an essential part of our everyday lives, powering everything from our smartphones to our cars. But have you ever wondered how they actually work? In this article, we will explore the inner workings of batteries and uncover the science behind their power.
At the heart of every battery is a chemical reaction. Batteries consist of two electrodes – a positive electrode called the cathode and a negative electrode called the anode. These electrodes are immersed in an electrolyte solution, which allows ions to move between them.
When a battery is connected to a device, a chemical reaction occurs at the electrodes. At the anode, electrons are stripped from the atoms and flow through an external circuit, creating an electric current. Meanwhile, at the cathode, ions react with the electrons and the electrolyte, completing the circuit.
Different types of batteries use different materials for their electrodes and electrolytes. For example, alkaline batteries commonly used in household devices have a zinc anode, a manganese dioxide cathode, and an alkaline electrolyte. On the other hand, lithium-ion batteries, which power our smartphones and laptops, use lithium compounds for both the anode and cathode.
The voltage and capacity of a battery depend on the materials used and the size of the electrodes. Higher voltage batteries have more cells connected in series, while higher capacity batteries have larger electrodes or more active material.
In conclusion, batteries work by harnessing chemical reactions to produce an electric current. Understanding how batteries work is not only fascinating but also crucial in developing more efficient and sustainable energy storage solutions for the future. So next time you use a battery-powered device, remember the incredible science happening inside that small, portable power source.
What Are the Different Types of Batteries?
Batteries are a fundamental component of our modern lives, powering everything from our smartphones to electric vehicles. But have you ever wondered about the different types of batteries and how they work? In this article, we will explore the various types of batteries commonly used today.
One of the most common types of batteries is the alkaline battery. These batteries are widely available and are often used in household electronics such as remote controls and flashlights. Alkaline batteries work by converting chemical energy into electrical energy through a chemical reaction between zinc and manganese dioxide.
Another popular type of battery is the lithium-ion battery. These batteries are commonly found in smartphones, laptops, and electric vehicles. Lithium-ion batteries are known for their high energy density and long cycle life. They work by moving lithium ions between two electrodes, typically made of lithium cobalt oxide and graphite.
Lead-acid batteries, on the other hand, are commonly used in vehicles and backup power systems. These batteries utilize a chemical reaction between lead and sulfuric acid to generate electricity. Lead-acid batteries are known for their low cost and ability to deliver high currents.
Nickel-cadmium (NiCd) batteries were once popular but have been largely replaced by newer technologies. These batteries work by utilizing a chemical reaction between nickel oxide hydroxide and metallic cadmium. NiCd batteries are known for their high discharge rate and ability to withstand extreme temperatures.
Other types of batteries include nickel-metal hydride (NiMH) batteries, which are commonly used in portable electronics, and zinc-carbon batteries, which are often found in low-drain devices such as clocks and remote controls.
In conclusion, there are various types of batteries available, each with its own unique characteristics and applications. Understanding the different types of batteries and how they work can help us make informed decisions when it comes to choosing the right battery for our devices.
How Are Batteries Made?
Batteries are an essential part of our daily lives, powering everything from our smartphones to our cars. But have you ever wondered how these small powerhouses are made? In this article, we will delve into the fascinating process of battery manufacturing.
The first step in battery production is the creation of the electrode materials. These materials, such as lithium or lead, are carefully chosen based on the type of battery being made. They undergo various chemical processes to ensure they have the desired properties and are ready for assembly.
Once the electrode materials are ready, they are coated onto a current collector, which is usually made of metal. This creates the positive and negative electrodes of the battery. The electrodes are then separated by a thin layer called the separator, which prevents them from coming into direct contact and causing a short circuit.
Next, the electrodes and separator are carefully stacked together to form what is known as a jelly roll. This jelly roll is then placed into a metal casing, which acts as the outer shell of the battery. The casing is sealed to prevent any leakage of the electrolyte, which is the substance that allows the flow of ions between the electrodes.
After the casing is sealed, the battery undergoes a process called formation. This involves charging and discharging the battery multiple times to activate its chemical reactions and improve its performance. Once the formation process is complete, the battery is thoroughly tested to ensure it meets the required specifications.
In conclusion, the process of manufacturing batteries involves several intricate steps, from preparing the electrode materials to assembling the battery and testing its performance. Understanding how batteries are made gives us a deeper appreciation for these small but powerful devices that play a crucial role in our modern world.
What Are the Components of a Battery?
Batteries are an essential part of our daily lives, powering everything from our smartphones to our cars. But have you ever wondered what exactly goes into making a battery work? In this article, we will explore the components of a battery and how they come together to generate electricity.
At the heart of every battery are two electrodes – a positive electrode (also known as the cathode) and a negative electrode (also known as the anode). These electrodes are made from different materials, such as lithium, lead, or zinc, depending on the type of battery. The electrodes are immersed in an electrolyte solution, which allows the flow of ions between them.
The electrolyte acts as a medium for the movement of ions, which are charged particles. When a battery is connected to a circuit, a chemical reaction occurs at the electrodes. At the positive electrode, ions lose electrons, creating a surplus of positive charge. These electrons flow through the circuit, creating an electric current. At the negative electrode, ions gain electrons, balancing out the charge.
To prevent the electrodes from coming into direct contact and causing a short circuit, a separator is placed between them. The separator allows the flow of ions while preventing the mixing of the electrodes.
In addition to these main components, batteries also have other elements such as current collectors, terminals, and casings. The current collectors help distribute the electric current evenly across the electrodes, while the terminals provide the connection points for the battery to be connected to a device or a circuit. The casing protects the internal components and ensures the battery remains intact.
Understanding the components of a battery is crucial in comprehending how batteries work. By harnessing the chemical reactions and the flow of ions, batteries are able to generate the electricity that powers our devices. Next time you use a battery-powered device, you’ll have a better understanding of the intricate components that make it all possible.
How Does a Battery Store and Release Energy?
Batteries are an essential part of our daily lives, powering everything from our smartphones to our cars. But have you ever wondered how a battery actually stores and releases energy? In this article, we will explore the fascinating world of battery technology and uncover the secrets behind their operation.
At its core, a battery is a device that converts chemical energy into electrical energy. It consists of two electrodes, an anode, and a cathode, separated by an electrolyte. When a battery is connected to a circuit, a chemical reaction occurs within the battery, causing electrons to flow from the anode to the cathode through the external circuit.
The anode is the negative terminal of the battery and is typically made of a metal such as zinc. When the battery is in use, zinc atoms oxidize, losing electrons and becoming positively charged ions. These ions then move through the electrolyte towards the cathode.
On the other hand, the cathode is the positive terminal of the battery and is usually made of a metal oxide. As the zinc ions reach the cathode, they react with the metal oxide, causing a reduction reaction that releases electrons. These electrons then flow through the external circuit, creating an electric current.
The electrolyte, which is usually a liquid or gel, acts as a medium for the movement of ions between the anode and cathode. It allows the chemical reactions to occur while preventing the direct contact of the two electrodes, which would result in a short circuit.
In conclusion, batteries store and release energy through a series of chemical reactions that involve the movement of electrons and ions. Understanding the inner workings of batteries helps us appreciate their importance in our everyday lives and opens up possibilities for future advancements in energy storage technology.
What Is the Role of Electrolytes in Batteries?
Electrolytes play a crucial role in the functioning of batteries. Understanding their role is essential to comprehend how batteries work and why they are such a vital component in various devices and applications.
In a battery, electrolytes are responsible for facilitating the movement of ions between the cathode and the anode. They act as conductive mediums that allow the flow of electric charge within the battery. This movement of ions is what generates the electrical current that powers our devices.
The electrolyte consists of a solvent, which can be a liquid or a solid, and dissolved ions. Typically, the solvent is a liquid, such as water or an organic solvent, while the dissolved ions are usually salts or acids. The choice of electrolyte depends on the specific battery chemistry and its intended application.
When a battery is connected to a circuit, a chemical reaction occurs at the electrodes. The electrolyte enables the transfer of ions between the electrodes, completing the circuit and allowing the flow of electrons. This flow of electrons creates an electric current that can be harnessed to power devices.
Furthermore, electrolytes also contribute to the overall stability and safety of the battery. They help prevent the accumulation of unwanted byproducts and regulate the internal temperature of the battery. In some cases, electrolytes may also participate in the electrochemical reactions, contributing to the overall energy storage capacity of the battery.
In conclusion, electrolytes are vital components in batteries, enabling the movement of ions and facilitating the flow of electric charge. Their role is crucial in generating the electrical current necessary to power our devices. Understanding the function of electrolytes is essential for further advancements in battery technology and the development of more efficient and sustainable energy storage systems.
What Causes Batteries to Die?
Batteries are an essential part of our daily lives, powering everything from our smartphones to our cars. But have you ever wondered what causes batteries to die? In this article, we will explore the factors that contribute to the demise of batteries and why they eventually lose their ability to hold a charge.
One of the main reasons batteries die is due to a chemical process called oxidation. Over time, the materials inside the battery react with the electrolyte, causing corrosion and degradation. This process is accelerated when the battery is exposed to high temperatures or stored in unfavorable conditions.
Another factor that affects battery life is the number of charge cycles. Each time a battery is charged and discharged, it goes through a cycle. With each cycle, the battery’s capacity to hold a charge decreases slightly. This is why batteries tend to last longer when they are cycled less frequently.
The type of battery also plays a role in its lifespan. Different chemistries have different characteristics and varying lifespans. For example, lithium-ion batteries, commonly found in smartphones and laptops, tend to have a limited lifespan of around 2-3 years. On the other hand, lead-acid batteries, used in cars and motorcycles, can last up to 5-7 years with proper maintenance.
External factors such as extreme temperatures can also impact battery life. Cold temperatures can reduce the battery’s capacity, while high temperatures can speed up the chemical reactions inside the battery, leading to faster degradation.
In conclusion, several factors contribute to the death of batteries, including oxidation, charge cycles, battery chemistry, and external conditions. Understanding these factors can help us prolong the lifespan of our batteries and make informed choices when it comes to using and storing them. So the next time you wonder why your battery is not holding a charge, remember that it’s a complex interplay of various factors that eventually cause batteries to die.
How Can Batteries Be Recycled?
Batteries play a vital role in our daily lives, powering everything from our smartphones to our cars. However, the improper disposal of batteries can have a detrimental impact on the environment. That’s why recycling batteries is crucial for the sustainability of our planet.
When it comes to recycling batteries, the process varies depending on the type of battery. Let’s take a closer look at how different batteries can be recycled.
1. Lead-Acid Batteries: These are commonly found in cars and are highly recyclable. The recycling process involves breaking down the battery into its components, such as lead, plastic, and sulfuric acid. These components are then separated and can be used to manufacture new batteries or other products.
2. Lithium-Ion Batteries: These batteries are commonly used in portable electronics. Recycling lithium-ion batteries involves dismantling them and separating the different materials, such as lithium, cobalt, and nickel. These materials can then be used to produce new batteries or other products.
3. Nickel-Cadmium Batteries: Although less common nowadays, nickel-cadmium batteries are still found in some devices. The recycling process for these batteries involves separating nickel and cadmium, which can be reused in various applications.
4. Alkaline Batteries: These are the standard household batteries used in devices like remote controls and flashlights. The recycling process for alkaline batteries involves sorting them by type and then processing them to recover metals like zinc and manganese.
5. Rechargeable Batteries: Rechargeable batteries, such as nickel-metal hydride (NiMH) and nickel-cadmium (NiCd) batteries, can be recycled through specialized programs. These batteries are collected, sorted, and processed to recover valuable metals.
By recycling batteries, we can prevent hazardous materials from ending up in landfills and reduce the need for extracting new resources. It’s essential to dispose of batteries properly and take advantage of recycling programs available in your area.
Remember, recycling batteries not only benefits the environment but also contributes to the conservation of valuable resources. Let’s all do our part in keeping our planet green by recycling batteries responsibly.
In conclusion, understanding how batteries work is essential in our modern world where we rely heavily on portable electronic devices and renewable energy sources. Throughout this post, we have explored various topics related to the keyword “how do batteries work” and gained valuable insights into their functioning.
We began by delving into the basic principles behind battery operation. We learned that batteries convert chemical energy into electrical energy through a process called electrochemical reaction. This energy is stored and released through the movement of electrons between the battery’s positive and negative terminals.
Furthermore, we explored the different types of batteries available in the market, ranging from alkaline batteries commonly used in household devices to lithium-ion batteries found in smartphones and electric vehicles. Each type has its own unique characteristics and applications.
Understanding how batteries are made was another important aspect covered in this post. We learned about the different components that make up a battery, including the anode, cathode, and electrolyte. These components work together to facilitate the movement of electrons and ions, enabling the battery to store and release energy efficiently.
The role of electrolytes in batteries was also discussed. Electrolytes play a crucial role in facilitating the movement of ions between the battery’s electrodes, allowing for the flow of electrical current.
We also explored the reasons behind battery degradation and eventual death. Factors such as internal resistance, self-discharge, and chemical reactions contribute to the gradual decline in a battery’s performance over time.
Lastly, we touched upon the importance of battery recycling. With the increasing demand for batteries, it is crucial to adopt sustainable practices to minimize environmental impact. Recycling batteries not only helps recover valuable materials but also reduces the release of harmful substances into the environment.
Looking ahead, we can expect advancements in battery technology, such as improved energy density and faster charging capabilities. These developments will revolutionize various industries, including transportation and renewable energy.
Thank you for taking the time to read this post. We hope you found it informative and insightful. We encourage you to leave any comments or feedback, as your input is valuable to us. Remember, understanding how batteries work empowers us to make informed choices and contribute to a greener future.


Leave a Reply
Want to join the discussion?Feel free to contribute!